It was a low-key week for the biotech sector with very few updates. Bigwig Gilead Sciences (NASDAQ:GILD) obtained FDA approval for the label expansion of Descovy, while Akcea Therapeutics, Inc. (NASDAQ:AKCA) signed a licensing deal with pharma major Pfizer.
Recap of the Week’s Most Important Stories:
Gilead's HIV Regimen Gets FDA Nod for Label Expansion: Gilead announced that the FDA approved a label expansion of its HIV treatment, Descovy (emtricitabine 200 mg and tenofovir alafenamide 25 mg tablets; F/TAF), as a prevention option. The agency approved the treatment for a pre-exposure prophylaxis (PrEP) indication. Descovy for PrEP is indicated to reduce the risk of sexually acquired HIV-1 infection in adults and adolescents weighing at least 35 kg, who are HIV-negative and at-risk for sexually acquired HIV, excluding individuals at-risk from receptive vaginal sex.
The FDA approved Gilead’s supplemental New Drug Application (sNDA) for Descovy under a Priority Review designation. The regimen was approved in the United States in 2016 for the treatment of HIV-1 infection in combination with other antiretroviral agents. The approval was based on encouraging data from the multi-year, global, phase III registrational study, DISCOVER, wherein Descovy demonstrated non-inferior efficacy and an improved bone and renal safety profile compared with Truvada in people at risk of sexually acquired HIV Infection.
Separately, Gilead announced that the New Drug Application (NDA) for its investigational, oral, selective JAK1 inhibitor, filgotinib, for the treatment of adults with rheumatoid arthritis (RA) has been submitted to the Japanese Ministry of Health, Labor and Welfare (MHLW).
ACADIA Presents Positive Data From Studies: ACADIA Pharmaceuticals Inc. (NASDAQ:ACAD) announced positive data from the mid-stage study, CLARITY, at the 2019 Psych Congress. The study was a 10-week, double-blind, placebo-controlled, two-stage sequential parallel comparison design (SPCD) study, which evaluated the efficacy, safety and tolerability of pimavanserin as an adjunctive treatment for major depressive disorder (MDD) in patients, who have had an inadequate response to SSRI or SNRI therapy. Based on secondary analyses from the phase II CLARITY study, adjunctive pimavanserin showed the potential to improve symptoms of sexual dysfunction experienced by MDD patients. Pimavanserin met the overall primary endpoint, the key secondary endpoint and seven of the eleven pre-specified additional secondary endpoints.
Verastem Gets Orphan Drug Designation for Copiktra: Verastem, Inc. (NASDAQ:VSTM) announced that the FDA has granted Orphan Drug designation to Copiktra (duvelisib) for the treatment of T-Cell lymphoma. Copiktra is already approved as a third or later-line treatment for chronic lymphocytic leukemia/small lymphocytic lymphoma and follicular lymphoma. A phase II study — PRIMO — is evaluating Copiktra as monotherapy in patients with relapsed or refractory peripheral T-cell lymphoma ("PTCL").
Additionally, Verastem, announced that its partner Yakult Honsha Co., Ltd. has dosed the first patient in a phase Ib Japanese bridging study evaluating Copiktra (duvelisib) in patients with relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma, following at least one prior therapy. The company and Yakult entered an exclusive licensing agreement in June 2018 to develop and commercialize Copiktra for the treatment, prevention or diagnosis of all oncology indications in Japan.
Akcea Soars on Licensing Deal With Pfizer: Shares of Akcea Therapeutics, Inc., a majority-owned affiliate of Ionis Pharmaceuticals, Inc. (NASDAQ:IONS) , soared after it announced a licensing deal with Pfizer Inc. (NYSE:PFE) . Both companies have entered a worldwide exclusive licensing agreement for AKCEA-ANGPTL3-LRx, an investigational antisense therapy being developed to treat patients with certain cardiovascular and metabolic diseases. The candidate is being evaluated in a phase II study in patients with type 2 diabetes, hypertriglyceridemia and non-alcoholic fatty liver disease (NAFLD).
Per the agreement, Pfizer is responsible for all development and regulatory activities, and costs beyond those associated with the ongoing phase II study. Akcea and Ionis will receive a $250-million upfront license fee, which will be split equally between the companies. The company will settle its $125-million obligation to Ionis in Akcea common stock. The companies are also eligible to receive development, regulatory and sales milestone payments of up to $1.3 billion and tiered, double-digit royalties on annual worldwide net sales following marketing approval of the candidate.
Akcea currently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Performance
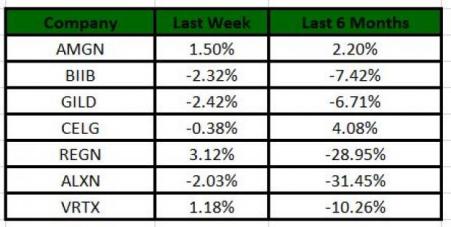
The Nasdaq Biotechnology index lost 0.44% in the last five trading sessions. Among the biotech giants, Gilead lost 2.42% in the period. Over the past six months, shares of Celgene (NASDAQ:CELG) have gained 4.08%, whereas the Alexion (NASDAQ:ALXN) stock has declined 31.45%. (See the last biotech stock roundup here: Biotech Stock Roundup: Pipeline Updates From REGN, BIIB, AMGN and More)
What's Next in Biotech?
Stay tuned for more pipeline updates.
Today's Best Stocks from Zacks
Would you like to see the updated picks from our best market-beating strategies? From 2017 through 2018, while the S&P 500 gained +15.8%, five of our screens returned +38.0%, +61.3%, +61.6%, +68.1%, and +98.3%.
This outperformance has not just been a recent phenomenon. From 2000 – 2018, while the S&P averaged +4.8% per year, our top strategies averaged up to +56.2% per year.
See their latest picks free >>
Pfizer Inc. (PFE): Free Stock Analysis Report
Verastem, Inc. (VSTM): Free Stock Analysis Report
ACADIA Pharmaceuticals Inc. (ACAD): Free Stock Analysis Report
Gilead Sciences, Inc. (GILD): Free Stock Analysis Report
Ionis Pharmaceuticals, Inc. (IONS): Free Stock Analysis Report
Akcea Therapeutics, Inc. (AKCA): Free Stock Analysis Report
Original post
Zacks Investment Research